This is best explained by Charles Kittel on page 53 of his book Introduction to Solid State Physics, 8th Edition: ``What holds an inert gas crystal together?...Consider two identical inert gas atoms at a separation
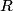 large in comparison with the radii of the atoms. What interactions exist between the two neutral atoms? If the charge distributions on the atoms were rigid, the interaction between atoms would be zero, because the electrostatic potential of a spherical distribution of electronic charge is canceled outside a neutral atom by the electrostatic potential of the charge on the nucleus. Then the inert gas atoms could show no cohesion and could not condense. But the atoms induce dipole moments in each other, and the induced moments cause an attractive interaction." Therefore, the correct answer is (E). It is Van der Waals bonding that holds the solid argon together. large in comparison with the radii of the atoms. What interactions exist between the two neutral atoms? If the charge distributions on the atoms were rigid, the interaction between atoms would be zero, because the electrostatic potential of a spherical distribution of electronic charge is canceled outside a neutral atom by the electrostatic potential of the charge on the nucleus. Then the inert gas atoms could show no cohesion and could not condense. But the atoms induce dipole moments in each other, and the induced moments cause an attractive interaction." Therefore, the correct answer is (E). It is Van der Waals bonding that holds the solid argon together. |